For this post, I feel compelled to talk about fishies.
 Okay, well, that fish is a robot. I'm talking about real fish. Specifically the kind that live under thick sheets of ice for months at a time. Didn't you ever wonder how they don't die?
Okay, well, that fish is a robot. I'm talking about real fish. Specifically the kind that live under thick sheets of ice for months at a time. Didn't you ever wonder how they don't die?Here in sunny Florida, it's been roughly 35F-40F all week, which is quite the shock to those avid beach goers. The snowbirds are probably thinking that they should have stayed up North. Except we don't have any snow.
But back to the fish thing.
There are actually a bunch of different ways the little masterminds avoid becoming popsicles.
Suppose you have a freshwater lake. In this case, we'll focus on the Physics of it. Typically, things expand as you heat them and contract when you cool them. Water, however, likes to be special. It does this weird parabola thing when you change the temperature.
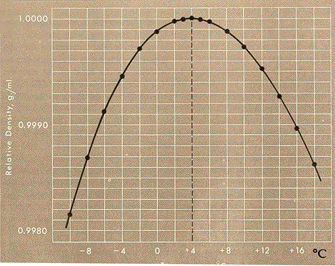 Basically, that graph says that water is most dense at 4C. So the water acts normally, getting more dense as it's cooled (why ice makes your water bottles stretch out). But it stops getting more dense at 4C. For the fish, this is a very good thing. Imagine, if you would, a lake.
Basically, that graph says that water is most dense at 4C. So the water acts normally, getting more dense as it's cooled (why ice makes your water bottles stretch out). But it stops getting more dense at 4C. For the fish, this is a very good thing. Imagine, if you would, a lake. With fish in it.
With fish in it. Now it starts to get colder. Say the lake is completely at a temperature of 5C in the beginning.
Now it starts to get colder. Say the lake is completely at a temperature of 5C in the beginning. Fish are a little chilly, but hey, it's the burden of a fish life. Then that top layer of water gets cooled to 4C. What happens? It's denser than it was at 5C. So it sinks to the bottom.
Fish are a little chilly, but hey, it's the burden of a fish life. Then that top layer of water gets cooled to 4C. What happens? It's denser than it was at 5C. So it sinks to the bottom. (The fish know it's beginning)
(The fish know it's beginning)SO the veeeery bottom of the lake is at 4C. This process keeps happening until the ENTIRE lake is at 4C. But then what? The top layer gets cooled to 3C. But since it's less dense than the rest of the water, according to our lovely parabola, that water stays up on top. Slooowly, that layer cools the next layer and that layer cools the next layer and so on. Then, once the top layer reaches 0C, the freezing point of water, the top of the lake freezes. At that point, though, the water at the bottom of the lake is still 4C.
 And so the fish get to not freeze.
And so the fish get to not freeze.In places like the Artic, where the fish ARE swimming in water below freezing point (because salt water can do that and not freeze), different stuff happens. Apparently, some of these fish have natural anti-freeze in their blood. From what I understand, it's actually some protein (insert name here) covered in sugar.
Everything is manipulated to keep the fishies happy.
Yup. Trufax.

Oh, Melissa. My life would be so void of physicsing if it wasn't for you.
ReplyDeletePhysics is ALWAYS there. You could never be void of it.
ReplyDelete^_~ BUT YOU KNOW YOU LOVE ME FOR THE PICTURES!!!